The dangerously blurry line between wellness and medical tech
-
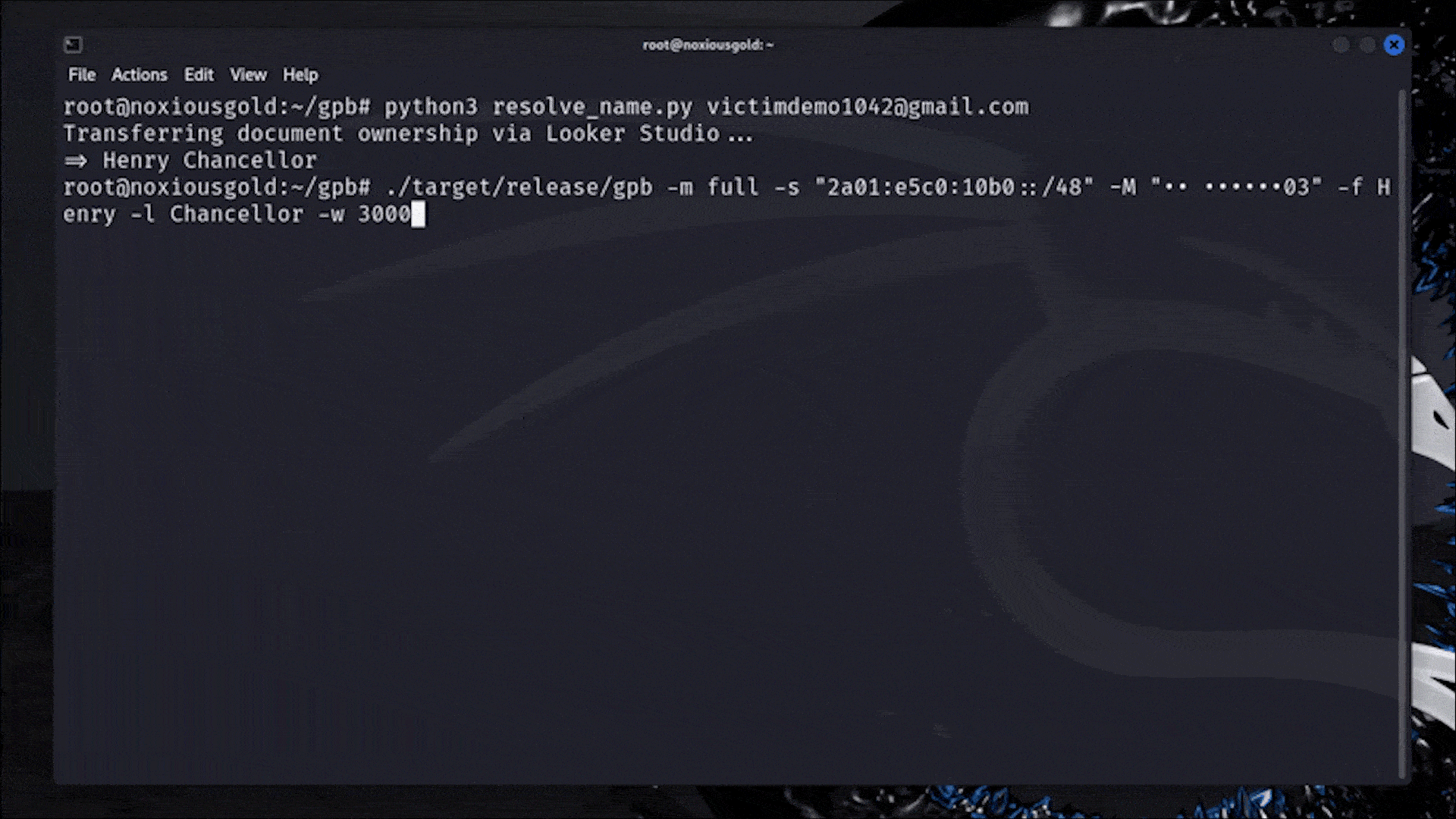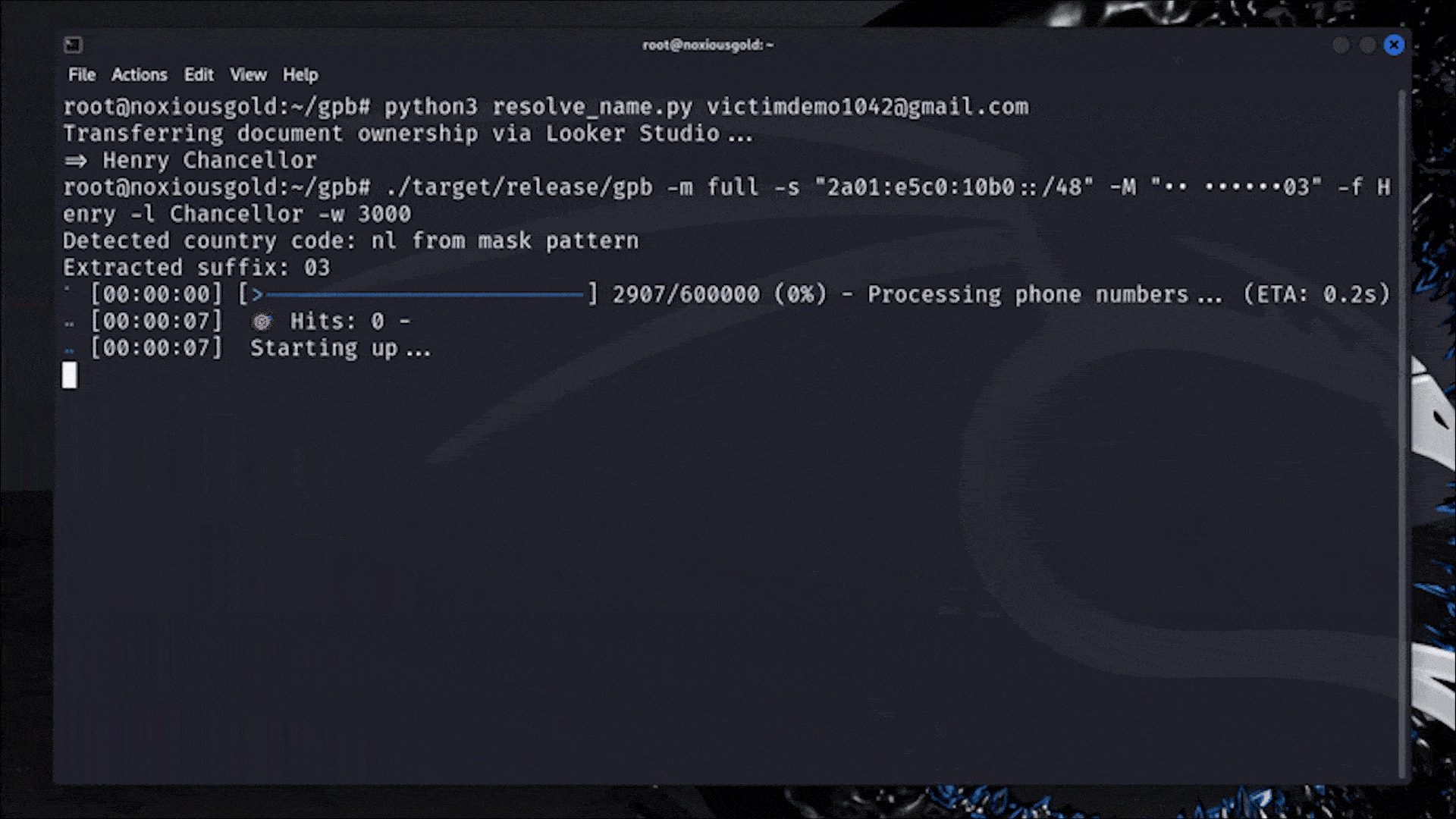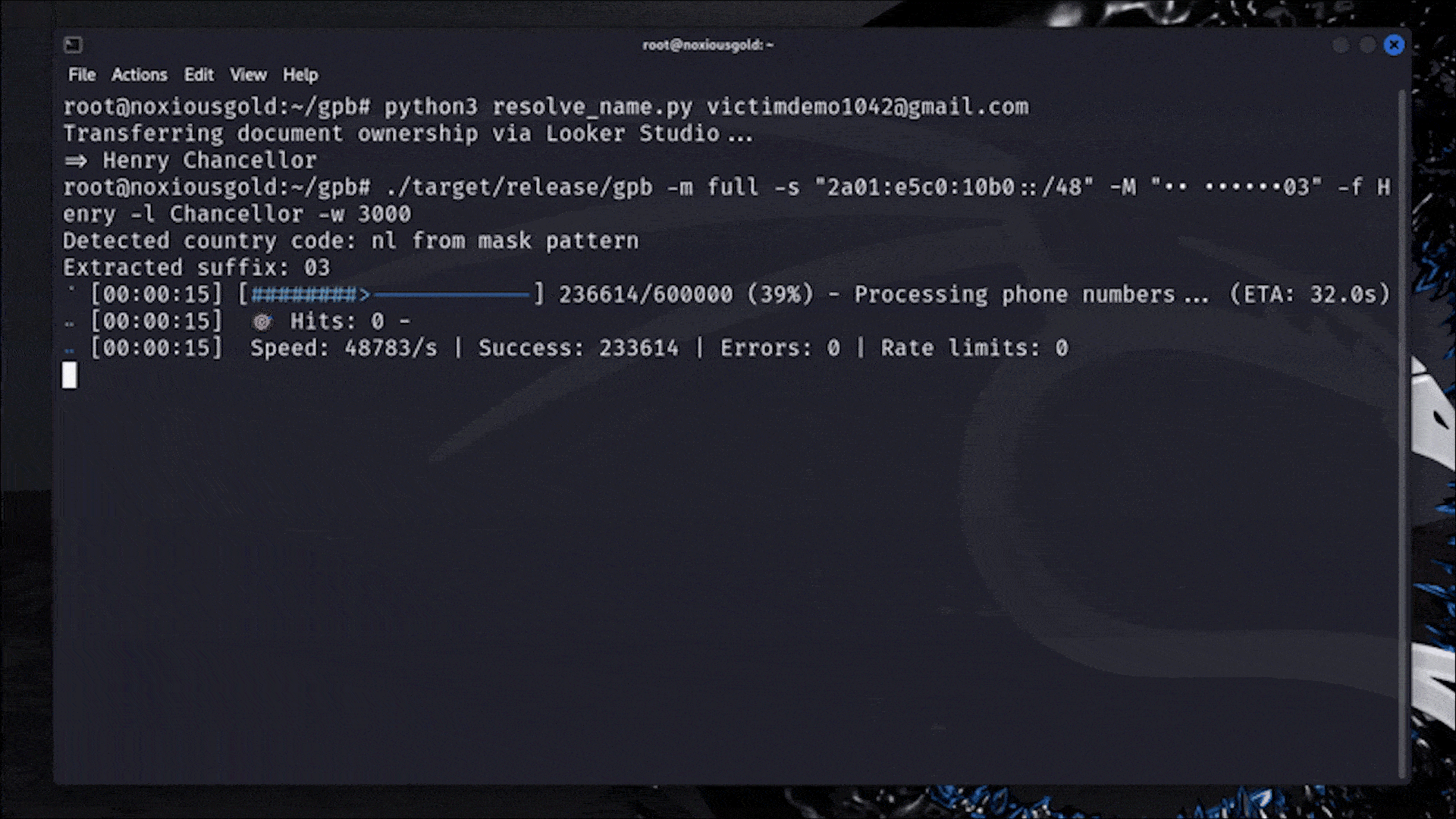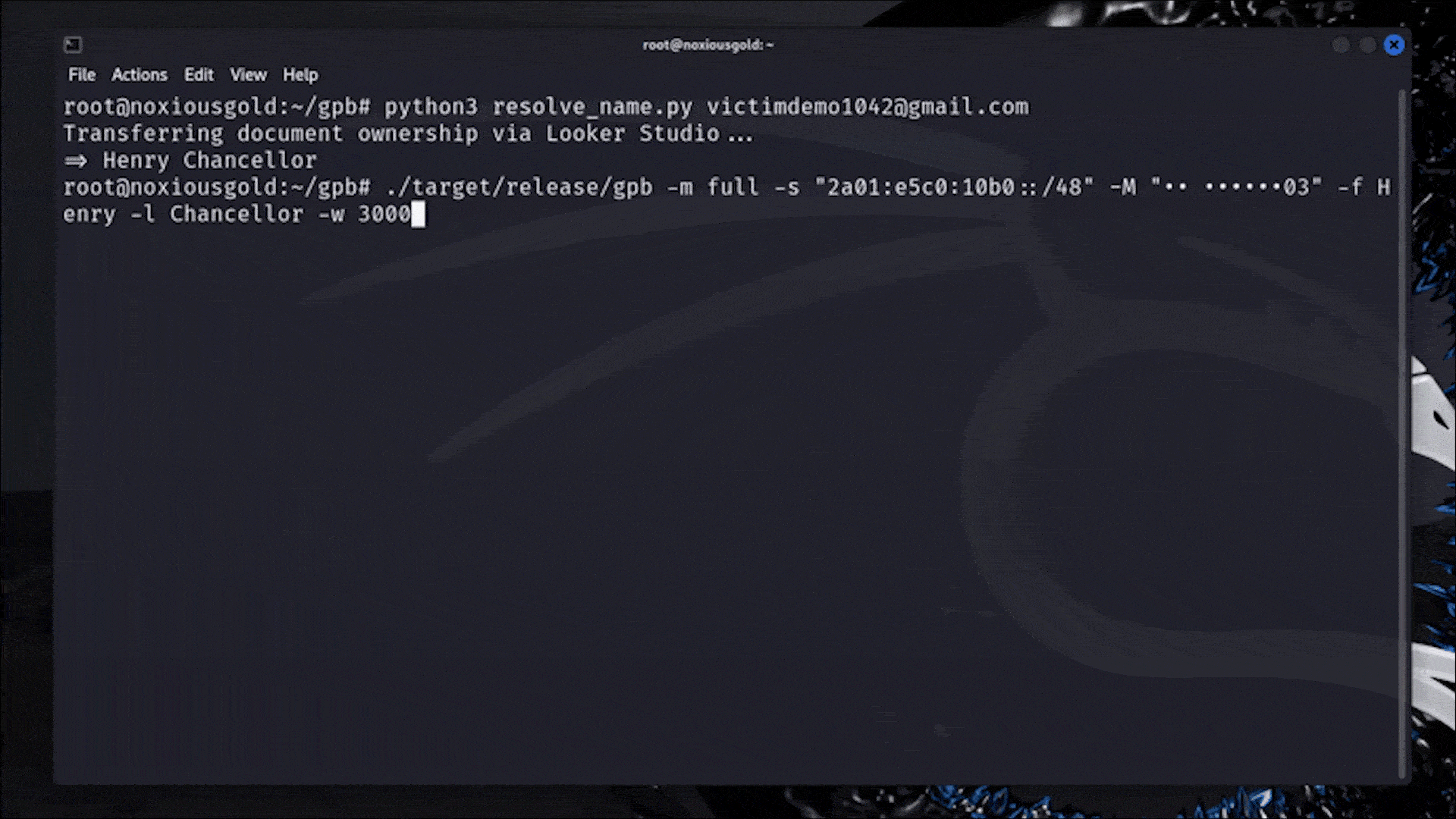
Let me catch you up. On July 15th, the Food and Drug Administration (FDA) sent Whoop a letter. In it, the FDA declared that Whoop — maker of a niche fitness tracker favored by elite athletes — had crossed a line. Its new Blood Pressure Insights feature, the regulator said, was being marketed to customers without undergoing the proper clearance process.
In response, Whoop pulled out the W word: wellness.
In the world of health and wearable tech, “wellness” is sort of like a “get out of jail free” card. Some advanced health features, like EKGs and atrial fibrillation notifications, require regulatory clearance before consumers can use them. These features could be interpreted as diagnostic in nature or prompt a person to make a medical decision. Others, like step tracking and blood oxygen measurements, don’t require FDA oversight at all. They’re simply meant to make living a healthy life easier by helping you visualize certain measurable markers. Those features fall under the wellness umbrella. They’re “just for fun.”
Archive: https://archive.is/IeGW2

The dangerously blurry line between wellness and medical tech
Whoop is saying its latest blood pressure insights feature is wellness tech. The FDA disagrees, saying it has clear medical and diagnostic implications.

The Verge (www.theverge.com)
-
Let me catch you up. On July 15th, the Food and Drug Administration (FDA) sent Whoop a letter. In it, the FDA declared that Whoop — maker of a niche fitness tracker favored by elite athletes — had crossed a line. Its new Blood Pressure Insights feature, the regulator said, was being marketed to customers without undergoing the proper clearance process.
In response, Whoop pulled out the W word: wellness.
In the world of health and wearable tech, “wellness” is sort of like a “get out of jail free” card. Some advanced health features, like EKGs and atrial fibrillation notifications, require regulatory clearance before consumers can use them. These features could be interpreted as diagnostic in nature or prompt a person to make a medical decision. Others, like step tracking and blood oxygen measurements, don’t require FDA oversight at all. They’re simply meant to make living a healthy life easier by helping you visualize certain measurable markers. Those features fall under the wellness umbrella. They’re “just for fun.”
Archive: https://archive.is/IeGW2

The dangerously blurry line between wellness and medical tech
Whoop is saying its latest blood pressure insights feature is wellness tech. The FDA disagrees, saying it has clear medical and diagnostic implications.

The Verge (www.theverge.com)
Subscription required to read

-
Subscription required to read

Woops. My bad.
-
Woops. My bad.
We were this close to perfection
-
Woops. My bad.
Ty for the archive.
-
Let me catch you up. On July 15th, the Food and Drug Administration (FDA) sent Whoop a letter. In it, the FDA declared that Whoop — maker of a niche fitness tracker favored by elite athletes — had crossed a line. Its new Blood Pressure Insights feature, the regulator said, was being marketed to customers without undergoing the proper clearance process.
In response, Whoop pulled out the W word: wellness.
In the world of health and wearable tech, “wellness” is sort of like a “get out of jail free” card. Some advanced health features, like EKGs and atrial fibrillation notifications, require regulatory clearance before consumers can use them. These features could be interpreted as diagnostic in nature or prompt a person to make a medical decision. Others, like step tracking and blood oxygen measurements, don’t require FDA oversight at all. They’re simply meant to make living a healthy life easier by helping you visualize certain measurable markers. Those features fall under the wellness umbrella. They’re “just for fun.”
Archive: https://archive.is/IeGW2

The dangerously blurry line between wellness and medical tech
Whoop is saying its latest blood pressure insights feature is wellness tech. The FDA disagrees, saying it has clear medical and diagnostic implications.

The Verge (www.theverge.com)
I am honestly surprised the FDA did anything here. Whoop should just wait a bit and see if there's any follow-up.
-
Ty for the archive.
You're welcome. I forgot The Verge sometimes paywalls it's articles.
-
Let me catch you up. On July 15th, the Food and Drug Administration (FDA) sent Whoop a letter. In it, the FDA declared that Whoop — maker of a niche fitness tracker favored by elite athletes — had crossed a line. Its new Blood Pressure Insights feature, the regulator said, was being marketed to customers without undergoing the proper clearance process.
In response, Whoop pulled out the W word: wellness.
In the world of health and wearable tech, “wellness” is sort of like a “get out of jail free” card. Some advanced health features, like EKGs and atrial fibrillation notifications, require regulatory clearance before consumers can use them. These features could be interpreted as diagnostic in nature or prompt a person to make a medical decision. Others, like step tracking and blood oxygen measurements, don’t require FDA oversight at all. They’re simply meant to make living a healthy life easier by helping you visualize certain measurable markers. Those features fall under the wellness umbrella. They’re “just for fun.”
Archive: https://archive.is/IeGW2

The dangerously blurry line between wellness and medical tech
Whoop is saying its latest blood pressure insights feature is wellness tech. The FDA disagrees, saying it has clear medical and diagnostic implications.

The Verge (www.theverge.com)
Have they tried a bribe? Seems to work pretty well.
-
We were this close to perfection
I know, where's that
h